- Atomic No Of Oxygen
- Atomic No Of Oxygen Elements
- How Many Atoms Are In O2
- How To Calculate Atomic Number Of Oxygen
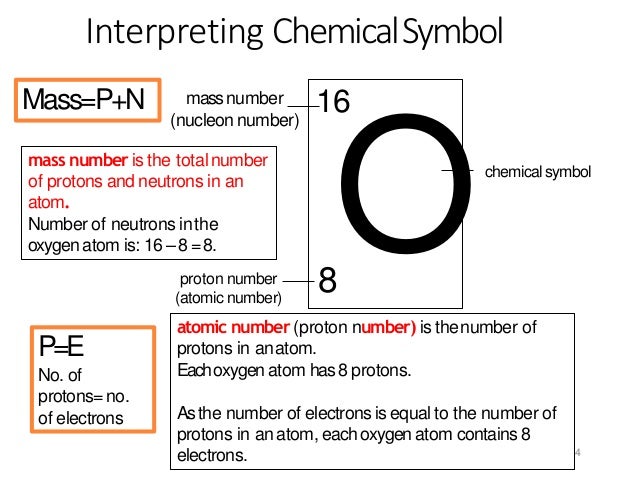
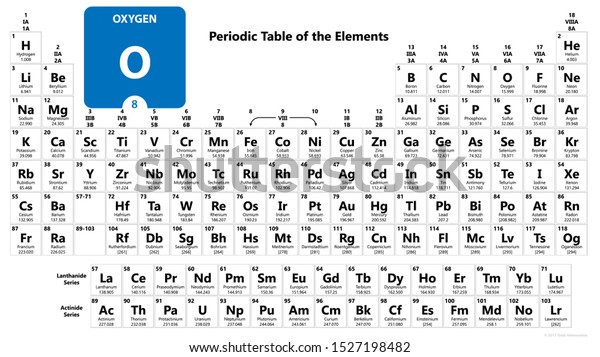
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). Oxygen is a chemical element with atomic number 8 which means there are 8 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. The atomic oxygen attacked silver surface (fig. 3a) indicate that the sulphur and chlorine layers are very thin, but that there is a carbonaceous layer of approxi-mately 50-100 nm thick followed by a layer which is essentially silver and oxygen of about 2 to 3urn in thickness. Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point:-218.4 °C (54.750008 K, -361.12 °F) Boiling Point:-183.0 °C (90.15 K, -297.4 °F) Number of Protons/Electrons: 8 Number of Neutrons: 8 Classification: Non-metal Crystal Structure: Cubic Density @ 293 K: 1.429 g/cm 3 Color: colorless Atomic Structure.
What is the mass number of oxygen?
2 Answers
Usb2.0 serial driver windows 7. The most common Isotope Mass for Oxygen is 16 Atomic Mass Units.
Explanation:
The Weighted Average Atomic Mass for each element is found on the periodic table (That long decimal number near the bottom of each box). This number is a combination of all the known Isotopes and basically tells you the average mass you would expect per atom if you grabbed a random handful of them.
The Mass Number is the mass of a particular atom individually and is measured in Atomic Mass Units (AMU); these will always be whole numbers.
The most common Isotope is found by rounding the Weighted average found on the periodic table to the nearest whole number.
In this case Oxygen has a Weighted Average Atomic Mass of 15.999 AMU. So the Mass Number is 16.
Explanation:
Atomic No Of Oxygen
Mass number
The most common isotope of oxygen has 8 neutrons. Its atomic number is 8.
This isotope of oxygen is oxygen-16.
Another isotope of oxygen has 9 neutrons.
This isotope is oxygen-17.
A third oxygen isotope has 10 neutrons.
This oxygen isotope is oxygen-18.
The following is the isotopic or nuclear notation of an isotope.
Atomic No Of Oxygen Elements

Related questions
What is the mass number of oxygen?
2 Answers
The most common Isotope Mass for Oxygen is 16 Atomic Mass Units.
Explanation:
The Weighted Average Atomic Mass for each element is found on the periodic table (That long decimal number near the bottom of each box). This number is a combination of all the known Isotopes and basically tells you the average mass you would expect per atom if you grabbed a random handful of them.
The Mass Number is the mass of a particular atom individually and is measured in Atomic Mass Units (AMU); these will always be whole numbers.
The most common Isotope is found by rounding the Weighted average found on the periodic table to the nearest whole number.
In this case Oxygen has a Weighted Average Atomic Mass of 15.999 AMU. So the Mass Number is 16.
Explanation:
Mass number
The most common isotope of oxygen has 8 neutrons. Its atomic number is 8. Skyrim female underwear replacer.
This isotope of oxygen is oxygen-16.
How Many Atoms Are In O2
Another isotope of oxygen has 9 neutrons. Belt conveyor design software, free download.
This isotope is oxygen-17.
How To Calculate Atomic Number Of Oxygen
A third oxygen isotope has 10 neutrons.
This oxygen isotope is oxygen-18.
The following is the isotopic or nuclear notation of an isotope.
Related questions
